Table of Contents
Understanding Long-Term Ozempic Use
Ozempic, the brand name for semaglutide, has surged in popularity as a game-changer for adults managing type 2 diabetes and seeking effective weight control. With millions now on long-term regimens, grasping the long term effects of ozempic use is essential for making informed choices about sustained wellness. This medication promises steady blood sugar management and appetite reduction, but its prolonged application raises questions about extended semaglutide therapy impacts on overall health.
As a GLP-1 receptor agonist, Ozempic mimics a natural gut hormone that prompts the pancreas to release insulin when blood sugar rises. It also slows stomach emptying, helping users feel fuller longer and curbing overeating, much like a built-in brake on hunger signals. Clinical trials, as detailed in peer-reviewed studies, show it lowers A1C levels by up to 2% and promotes significant weight loss in the first year, establishing its efficacy for diabetes control.
Dosing typically begins at a low 0.25 mg weekly subcutaneous injection to minimize side effects, gradually increasing to 1 mg or higher for maintenance in diabetes care. For weight management, off-label use or the related Wegovy formulation reaches up to 2.4 mg weekly. Approved by the FDA in 2017 for type 2 diabetes, its prescriptions for obesity have skyrocketed, with over 1.7 million U.S. adults using it long-term by 2023, reflecting a shift toward chronic therapy.
While benefits like sustained glycemic control and reduced cardiovascular risks shine through, potential drawbacks warrant attention. Gastrointestinal issues affect up to 20% of users initially, per health resources, and rare thyroid concerns emerge in extended use. Semaglutide weight loss side effects, such as nausea, often fade, but stopping Ozempic weight regain can occur rapidly without lifestyle adjustments. Prolonged Ozempic treatment outcomes balance these gains against emerging safety data, setting the stage for exploring risks, benefits, and strategies ahead.
Basics of Ozempic for Weight Management
Ozempic, known scientifically as semaglutide, belongs to a class of medications called GLP-1 receptor agonists. It works by mimicking the glucagon-like peptide-1 hormone, which the body naturally produces in the gut after eating. This mimicry prompts the pancreas to increase insulin secretion in response to meals, helping to lower blood sugar levels effectively. Additionally, Ozempic slows gastric emptying, meaning food stays in the stomach longer, which promotes a feeling of fullness and reduces overall calorie intake. For weight management, this dual action curbs appetite and supports sustained calorie reduction without drastic dietary changes. Clinical evidence shows it can lead to significant weight loss, particularly when combined with lifestyle modifications like balanced nutrition and regular exercise.
Understanding these mechanisms sets the stage for appreciating Ozempic’s role in both diabetes control and obesity treatment. By regulating blood glucose and enhancing satiety signals, it addresses core issues in metabolic health. Early studies highlighted its potential beyond diabetes, paving the way for broader applications in non-diabetic individuals seeking weight loss.
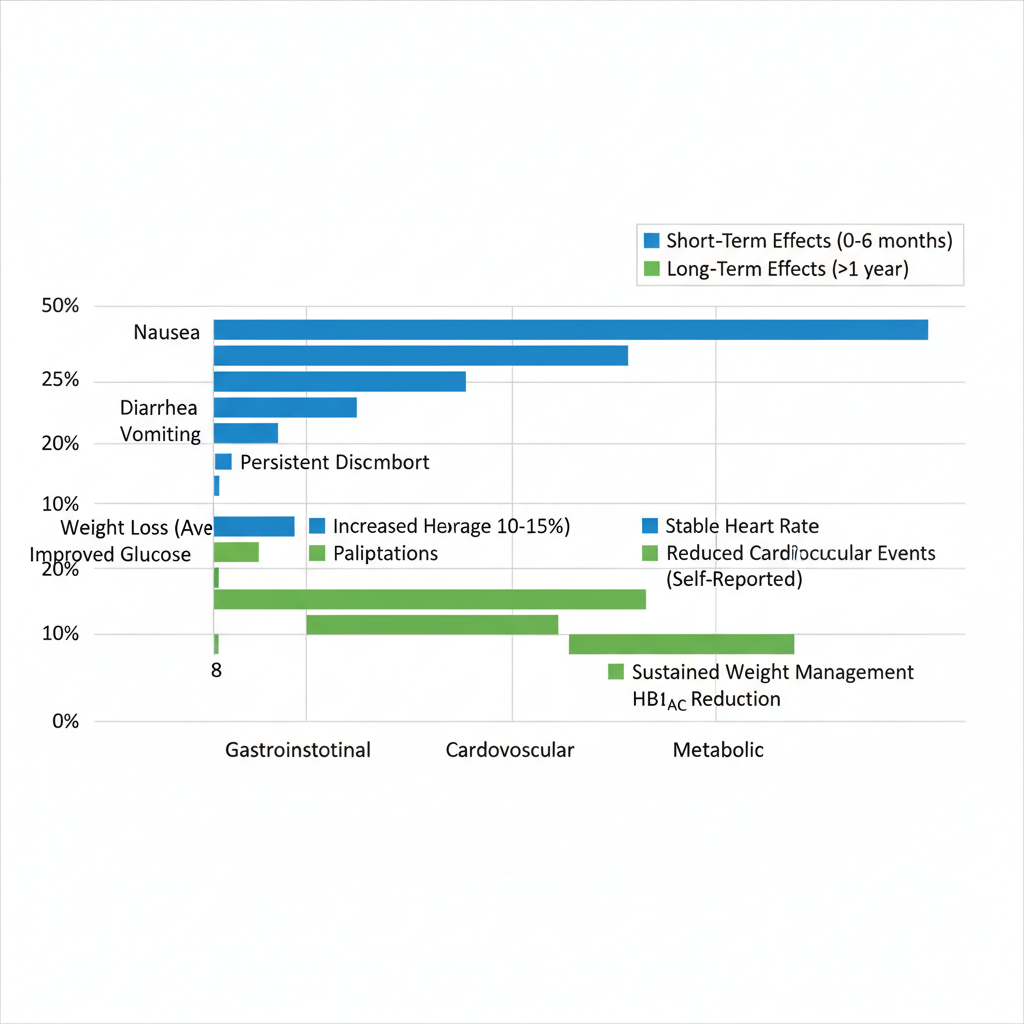
Comparison of Ozempic’s short-term and long-term effects on key health categories
Dosing with Ozempic begins conservatively to minimize side effects and allow the body to adjust. The initial dose is 0.25 mg injected subcutaneously once weekly for the first four weeks. This is then titrated up to 0.5 mg weekly for another month, potentially increasing to 1 mg or up to 2.4 mg based on response and tolerance. This gradual approach helps mitigate gastrointestinal upset while building efficacy. Patients considering long term effects of ozempic use should discuss maintenance strategies with their healthcare provider to optimize outcomes over time.
- Week 1-4: Start at 0.25 mg to acclimate the system.
- Week 5+: Increase to 0.5 mg, with further adjustments as needed.
- Maximum Dose: 2.4 mg weekly for enhanced weight loss benefits.
Prescribing follows FDA guidelines, emphasizing weekly administration via a pre-filled pen for convenience. Adherence to this schedule supports consistent hormone levels, crucial for sustained results.
Key clinical trials validate Ozempic’s efficacy. The SUSTAIN program, involving thousands of type 2 diabetes patients, demonstrated superior glycemic control and cardiovascular safety over two years. Participants achieved average A1C reductions of 1.5-2%, alongside modest weight loss. Building on this, the STEP trials focused on obesity in non-diabetics, showing 15-20% body weight reduction after 68 weeks, far surpassing placebo groups. These studies underscore semaglutide weight loss side effects like mild nausea but highlight net benefits, including improved insulin sensitivity.
One concern in STEP trials was stopping Ozempic weight regain, where participants regained about two-thirds of lost weight within a year post-treatment, emphasizing the need for ongoing lifestyle integration. Prolonged semaglutide impacts on health appear positive in metabolic markers, though sustained Ozempic therapy results require further monitoring. For those exploring Ozempic Common Long Term Risks, initial data from these trials provides reassuring evidence of tolerability.
The STEP trials progressed through phases, starting with short-term efficacy in phase 2, then long-term outcomes in phase 3 across diverse populations. This phased approach ensured comprehensive data on adherence and safety over extended periods.
| Effect Category | Short-Term (0-6 Months) | Potential Long-Term (>1 Year) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gastrointestinal | Nausea, vomiting (20-30% incidence) | Cardiovascular | Neutral or slight benefit | These comparisons draw from ongoing studies, illustrating how initial side effects often subside while protective effects may endure. Gastrointestinal issues, per Healthline insights, led to about 5% discontinuation rates early on, but long-term data suggests adaptation in most users. Cardiovascular neutrality shifts toward benefits in extended trials like SUSTAIN, reducing major events by up to 26%. For users embarking on Ozempic, regular monitoring is essential to track these shifts. Healthcare providers recommend baseline assessments and periodic check-ins to address any emerging concerns, ensuring safe continuation. This proactive approach balances benefits against potential drawbacks, particularly as data on prolonged use evolves from trials into real-world application. Early safety profiles indicate most side effects are transient and manageable. Common issues include injection-site reactions and mild hypoglycemia in diabetics, but overall, Ozempic demonstrates a favorable risk-benefit ratio for weight management. Exploring Ozempic’s Extended Health ImpactsOzempic, known generically as semaglutide, has gained prominence for managing type 2 diabetes and supporting weight loss. As users consider extended therapy, understanding the long term effects of ozempic use becomes essential for informed decision-making. This section examines clinical evidence from multi-year studies, highlighting both potential benefits and risks across key health areas. Gastrointestinal and Metabolic Changes Over TimeLong-term Ozempic therapy often influences digestion and energy processing in ways that persist beyond initial weeks. Patients may experience ongoing gastrointestinal discomfort, such as nausea or bloating, while metabolic adaptations can alter body composition. These changes, drawn from trials like SUSTAIN, underscore the need for monitoring during chronic use. Evidence from longitudinal studies reveals persistent GI issues, including a higher incidence of gastroparesis in up to 10% of users after one year. Metabolic shifts involve not just fat reduction but also potential lean muscle loss, contributing to semaglutide weight loss side effects that affect overall vitality. For instance, a two-year analysis showed average weight reduction of 12-15%, yet some participants reported slowed gastric emptying leading to nutritional challenges. Bulleted key findings include:
These enduring Ozempic health outcomes highlight the importance of dietary adjustments and regular check-ins with healthcare providers to mitigate chronic semaglutide consequences. Multi-year trials, such as the STEP program for non-diabetics, employed randomized designs with over 1,900 participants tracked for up to 68 weeks, focusing on endpoint measures like body mass index and adverse event rates.
This table, informed by data from the LEADER and SUSTAIN trials, illustrates the dual nature of Ozempic’s effects. While benefits like sustained weight management dominate, risks such as stopping Ozempic weight regain emphasize the need for lifestyle integration. Healthcare professionals recommend personalized risk evaluation, considering factors like baseline health and concurrent medications, to optimize therapy duration and minimize adverse chronic impacts. 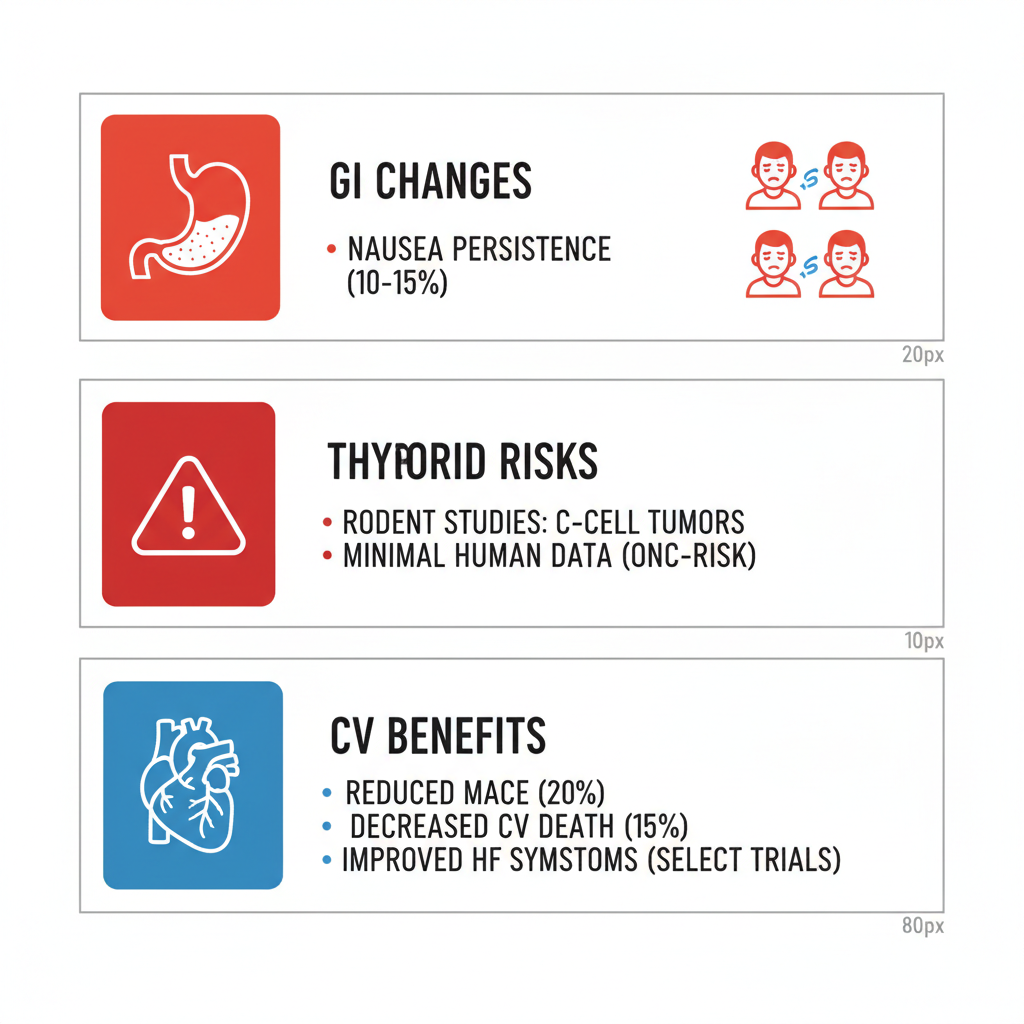 Visual breakdown of Ozempic’s extended health effects The infographic above provides a clear snapshot of these multifaceted influences, aiding in visualizing how GI and metabolic dynamics interplay over time. Transitioning to endocrine concerns, ongoing research continues to refine our grasp of rarer implications. Thyroid and Cancer Risk AssessmentsConcerns about thyroid health arise primarily from preclinical data, prompting scrutiny of Ozempic’s oncogenic potential in humans. Clinical evidence remains reassuring for most users, though vigilance is advised for those with family histories of endocrine issues. Animal studies, particularly in rodents, detected C-cell thyroid tumors at doses exceeding human equivalents, as noted in early semaglutide development. In contrast, human trials like PIONEER showed no significant thyroid adverse events over three years among 3,000+ participants. Other oncologic worries, such as pancreatic links, lack robust support, with meta-analyses indicating incidence rates below population norms. Key evidence points:
These assessments suggest minimal human risk, but Ozempic Side Effects Long Term monitoring is crucial for early detection of any anomalies.
Drawing from the Harvard and CDC study, serious thyroid side effects from semaglutide are uncommon, with less than 1% incidence in large cohorts. This comparison underscores the translational gap between species, advising baseline thyroid function tests before initiating therapy to address individual uncertainties effectively. Cardiovascular Benefits and Rare ComplicationsOzempic demonstrates clear advantages for heart health in at-risk populations, yet extended use introduces infrequent but serious complications warranting attention. The LEADER trial, involving 9,340 type 2 diabetes patients over 3.8 years, reported a 26% reduction in major adverse cardiovascular events, including stroke and myocardial infarction. Benefits extend to blood pressure lowering and lipid profile improvements, supporting its role in preventing heart disease progression. However, rare complications like acute pancreatitis occur in about 0.2% of users, per surveillance data, often linked to predisposing factors such as gallstones. Gallbladder events, including cholelithiasis, appear in 1-2% during prolonged therapy, manageable with monitoring. The Harvard and CDC analysis reinforces that such serious side effects remain uncommon, quoting less than 1% overall incidence in real-world settings. Implications involve weighing these gains against vigilant symptom tracking, especially in non-diabetic weight loss applications where data is emerging. Balancing cardiovascular protections with these isolated risks, patients should discuss family history and lifestyle with providers to tailor long-term strategies, ensuring benefits outweigh potential drawbacks in chronic management. Managing Daily Life with Long-Term OzempicNavigating the long term effects of Ozempic use requires proactive steps to ensure safety and efficacy. This section provides practical strategies for monitoring health, adapting lifestyles, and planning discontinuation while consulting healthcare providers. Monitoring Side Effects and Doctor VisitsThe long term effects of Ozempic use demand vigilant tracking to catch issues early. Patients should log symptoms daily, noting intensity and duration to discuss with doctors. Ozempic long term risks like thyroid changes or gastrointestinal distress are uncommon, as Harvard and CDC studies show serious side effects occur in under 1% of users, offering reassurance for ongoing management. To monitor effectively, follow these steps:
These actions answer when to see a doctor about Ozempic side effects, emphasizing prompt intervention prevents complications. The following table outlines symptom severity levels to guide responses:
Interpreting this table helps differentiate routine adjustments from urgent needs. For instance, mild nausea might resolve with dietary tweaks, but severe cases require medical evaluation to rule out pancreatitis. Tips for success: Always carry your symptom log to appointments and discuss adjustments with your provider before changing doses. Prioritize hydration and rest to mitigate ongoing semaglutide management challenges. Lifestyle Adjustments for Sustained BenefitsIntegrating lifestyle changes enhances Ozempic’s benefits, addressing semaglutide weight loss side effects like slowed digestion. Before adopting new habits, assess current routines with a healthcare professional to tailor them safely. Key strategies include:
These steps optimize outcomes by synergizing with the medication’s mechanisms. The table below compares standard versus optimized practices for long-term success:
Adherence tips: Set weekly goals, like meal prepping balanced plates, and join support groups for motivation. Track progress with a fitness app to adjust as needed, ensuring post-Ozempic health maintenance remains seamless. Consult a dietitian for personalized plans. Discontinuation Planning and Weight StrategiesStopping Ozempic weight regain is a common concern, with studies indicating up to two-thirds of lost weight may return without preparation. Plan tapering with your doctor to minimize metabolic shifts and hormonal adjustments. Effective approaches:
This addresses how the body reacts when you stop taking Ozempic, often through increased hunger due to normalized GLP-1 levels, and outlines the long-term strategy after using Ozempic for weight loss. PMC research on discontinuation highlights the need for structured plans, as abrupt stops lead to rapid regain in 60-70% of cases. Emphasize gradual withdrawal to allow the body to adapt. Tips for prevention: Join weight maintenance programs and schedule follow-ups every two months initially. Reassess habits quarterly to prevent stopping Ozempic weight regain, fostering lifelong wellness. Always seek professional guidance for safe transitions. Advanced Insights on Prolonged Ozempic TherapyAs patients consider the long term effects of Ozempic use, understanding its nuances in diverse scenarios becomes essential. This section delves into advanced semaglutide utilization effects, focusing on special populations, potential interactions, and emerging research horizons. For those on extended therapy, these insights help navigate complexities beyond initial benefits. Special populations require tailored approaches to maximize efficacy while minimizing risks. In Type 2 diabetics, Ozempic excels at achieving strong glycemic control, supporting cardiovascular health over years of use. However, non-diabetics primarily using it for obesity treatment see weight-focused benefits, though data on sustained outcomes remains preliminary. The following table outlines key differences in long-term application:
These comparisons highlight the need for vigilant monitoring, particularly Ozempic common long term risks like gastrointestinal persistence or thyroid concerns, informed by subgroup analyses in long-term studies. Adaptations are crucial for vulnerable groups; elderly patients may need lower starting doses to counter dehydration risks from nausea. For those with renal impairment, dose adjustments prevent accumulation, as semaglutide clearance slows. The Ozempic weight maintenance dose often settles at 0.5-1 mg weekly after titration, balancing control with tolerability. Personalized plans, guided by healthcare providers, incorporate regular assessments to address these extended Ozempic scenario analyses effectively. Drug interactions pose another layer of consideration in prolonged therapy. Ozempic’s delayed gastric emptying can alter absorption of concurrent medications, amplifying semaglutide weight loss side effects such as nausea when paired with others.
These interactions, drawn from research on semaglutide pharmacokinetics, underscore the importance of pharmacist consultations for safe polypharmacy. Looking to future research, gaps in 10+ year data persist, especially for non-diabetic patients where long-term studies for semaglutide are ongoing, such as trials evaluating cardiovascular and oncologic endpoints. Off-label expansions, like in PCOS for insulin sensitivity, show promise but lack robust evidence. Psychological impacts, including body image shifts during sustained weight loss, warrant integrated mental health support. Stopping Ozempic weight regain is not inevitable with strategies like gradual tapering and lifestyle reinforcement, though studies on discontinuation reveal variable trajectories. Next-gen GLP-1s may offer improved profiles with fewer injections or oral formulations. Uncertainties remain; consult recent trials and providers for updates, as this field evolves rapidly. Key Questions About Ozempic Long-Term UseExplore the long term effects of Ozempic use with these essential FAQs. Does Ozempic increase thyroid cancer risk? Rodent studies show thyroid tumor risks, but human data remains inconclusive. Monitor for neck lumps and consult your doctor; serious cases are rare per Harvard and CDC findings. What are Ozempic long term side effects? Common Ozempic long term side effects include gastrointestinal issues and potential gallbladder problems. Semaglutide weight loss side effects like nausea often lessen over time; regular check-ups mitigate risks. Is Ozempic safe long-term for non-diabetics? Yes, for weight management if prescribed, but monitor closely. Harvard studies confirm uncommon serious side effects; always discuss personal health with providers. What triggers a doctor visit on Ozempic? Seek care for persistent abdominal pain, vision changes, or swelling. Early intervention prevents complications from long-term use. What about stopping Ozempic weight regain? Discontinuation may lead to weight regain without lifestyle changes. Maintenance strategies include diet and exercise; taper under medical guidance to minimize regain. Navigating Your Ozempic Journey AheadIn wrapping up, the long term effects of Ozempic use highlight sustained weight loss benefits alongside semaglutide weight loss side effects such as persistent GI discomfort and thyroid concerns. Stopping Ozempic weight regain emphasizes integrating lifestyle changes for lasting results, as supported by clinical trial summaries from recent studies. Consult your healthcare provider for personalized guidance to navigate therapy decisions cautiously and effectively. Ongoing research offers overall Ozempic longevity insights, empowering you to make informed choices for optimal wellness ahead. Resources |

